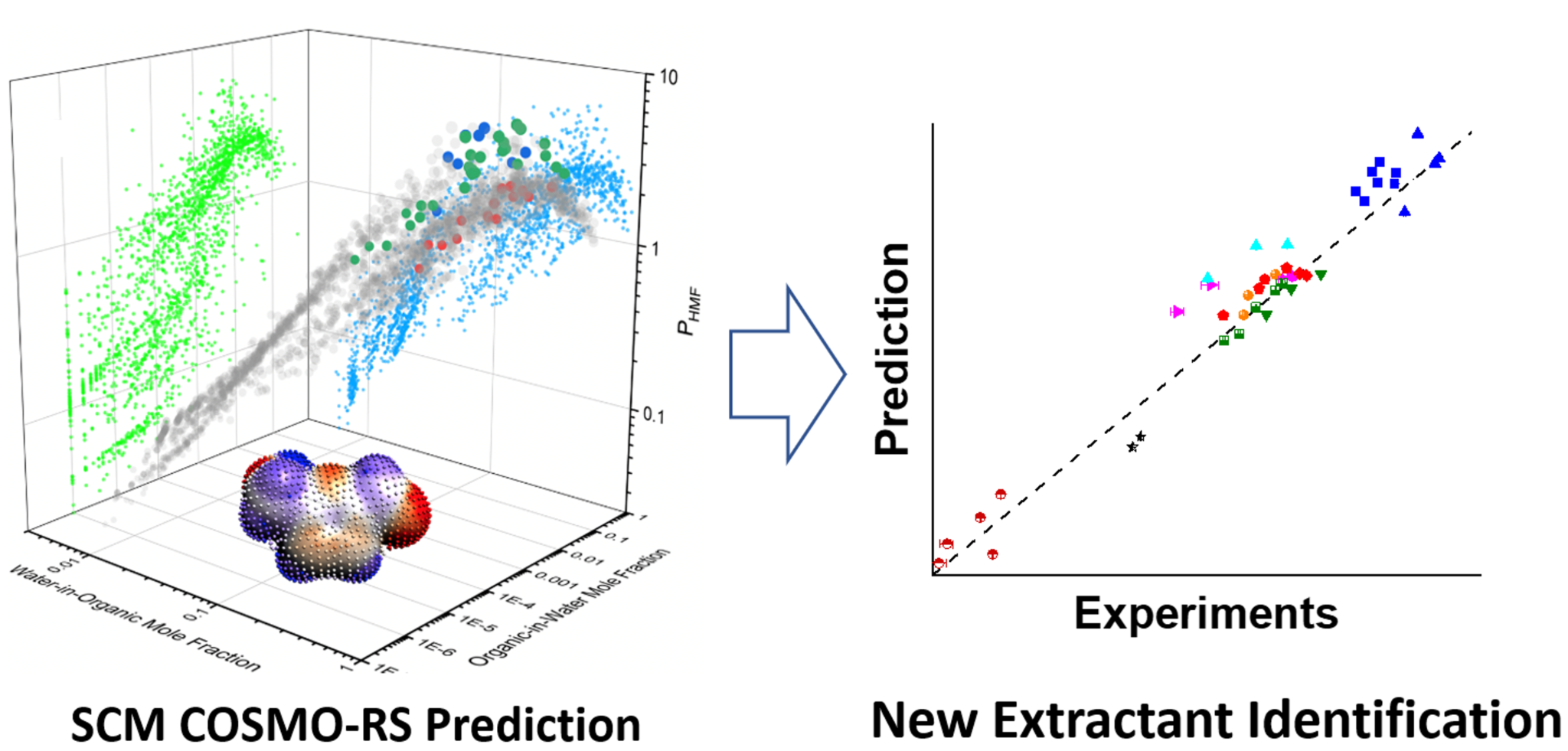
Renewable lignocellulosic biomass-derived platform chemicals, such as furfural and 5-hydroxymethylfurfural (HMF), offer promising pathways to tomorrow’s polymers, fine chemicals, and pharmaceuticals. High HMF yield from fructose dehydration in the aqueous phase is severely curtailed by side-reactions, which can be minimized by extracting HMF from the reactive aqueous phase into an organic phase. However, the selection of desirable solvents is far from trivial.
To improve solvent screening efficiency, researchers at the University of Delaware recently combined COSMO-RS predictions with targeted experiments to investigate the extraction of HMF in the fructose dehydration reaction. Aqueous-organic liquid-liquid equilibria at 298 K and 423 K (representative lignocellulosic biomass reaction temperature) are first predicted for all potential water-solvent pairs in the ADFCRS-2018 database. When a miscibility gap exists, equilibrium phase compositions are used to initialize logP calculations for HMF, levulinic acid (LA), and formic acid (FA), encountered in the fructose dehydration reaction. The partition coefficients were experimentally measured for solvents from multiple homologous series to evaluate the accuracy in the COSMO-RS predictions. Typical errors within a factor of ~2 demonstrate that the approach is excellent for screening purposes. For the first time, high-temperature partition coefficients for these species were also measured in situ. This investigation identified new classes of high performing extractants, such as substituted amines and phenols, for reactive extraction of HMF, with an experimentally verified order of magnitude improvement in partition coefficients over conventional extractants. Other criteria, such as solvent thermal stability, reactivity, and toxicity, were also evaluated. For more details, read the full paper in Green Chemistry.
Reference
- Zhaoxing Wang, Souryadeep Bhattacharyya, Dionisios G. Vlachos, Solvent Selection for Biphasic Extraction of 5-Hydroxymethylfurfural via Multiscale Modeling and Experiments, Green Chem., 22, 8699-8712 (2020). *Equal contribution
- SCM Newsletter – In Silico Solvent Selection for Lignocellulosic Biomass Conversion
